AleaSoft, August 7, 2019. Hydrogen and its use as an energy source have played a very important role in space travel. From AleaSoft, how hydrogen was used in the Apollo program that led the first human being to step on the Moon and its role in the current energy transition is reviewed.
In a recent news about the conquest of space and the photovoltaic revolution, AleaSoft described the role that some technologies that lead the energy transition, such as photovoltaics, had in the conquest of space. In this case, we are going to cover another technology with a strong presence in space travel: hydrogen. Surely not having an associated recognisable mechanical structure, such as the case of solar panels with photovoltaics, made its presence and use in space missions more unnoticed. But not for that reason, its role has been less important.
Hydrogen gas is the simplest molecule in the universe composed by two hydrogen atoms, the lightest element of the periodic table. In terrestrial conditions of temperature and pressure it exists in gaseous state and to turn it into a liquid it must be cooled to -253 °C, very close to absolute zero. But for its use as an energy source, its most interesting feature is its ability to combine with oxygen and release energy.
Hydrogen on the journey that led human beings to step on the Moon
Now that the fiftieth anniversary of the first manned trip that set foot on the Moon is celebrated, it is interesting to mention that, among other technologies, that trip was made possible by hydrogen. The most visible participation of hydrogen in the Apollo 11 mission was its use as a fuel for the Saturn V rockets. For the first stage of the rocket launch, when it rises above the ground in the middle of a fireball, the five F-1 engines of Saturn V used kerosene and oxygen as fuels. But for the second and third stages, the J-2 engines used hydrogen and oxygen, and were responsible for putting the probe into orbit and giving the final thrust that sent the vehicle to the Moon.
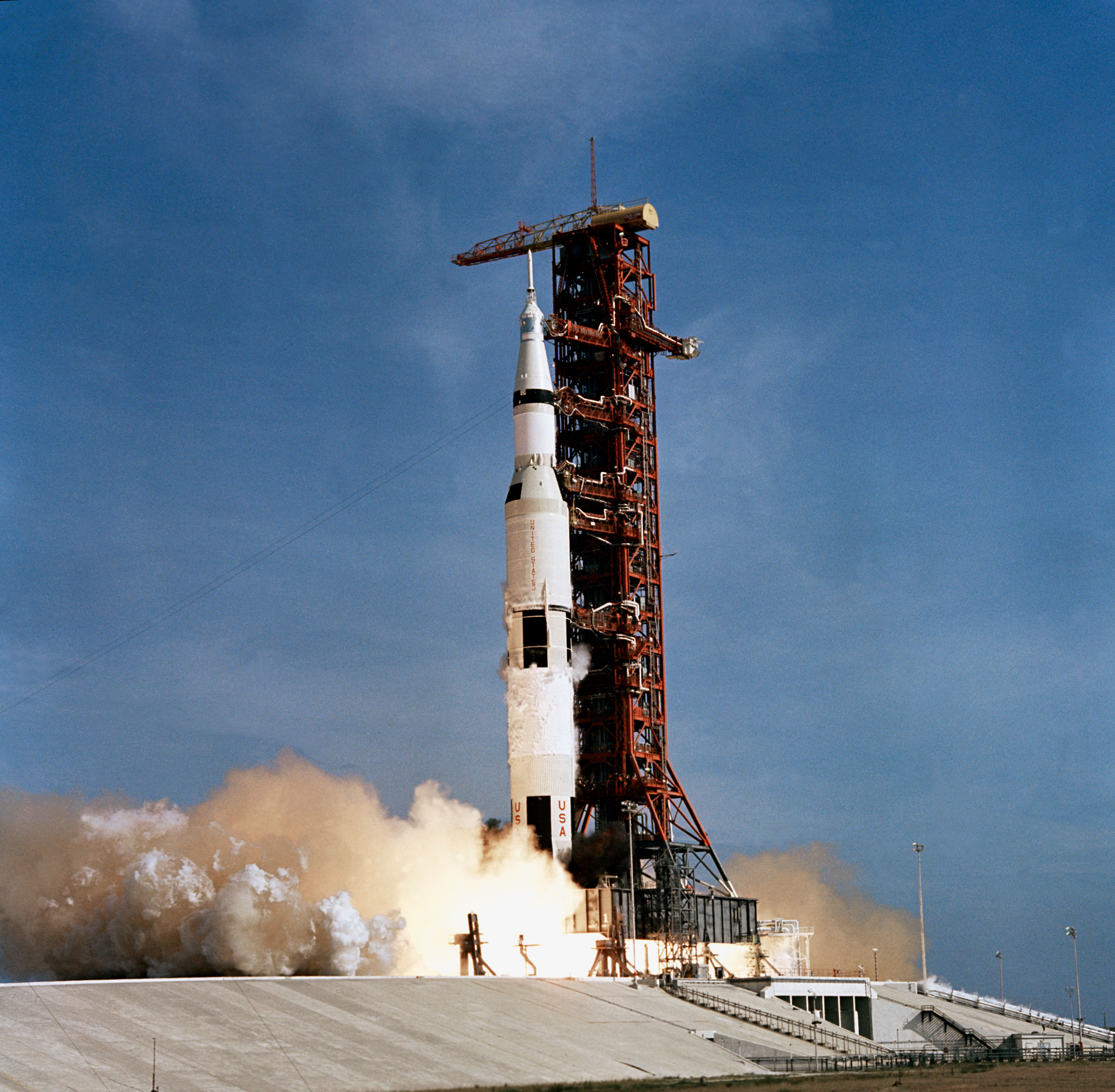
Launch of the Saturn V rocket with the Apollo 11 mission aboard. Source: NASA.
For rocket propulsion, what is used is the ability of hydrogen to be burned like a fossil fuel and produce an explosion, but with the important difference that during its combustion CO2 is not generated, only H2O, that is, water. Hydrogen can be used as fuel basically with the same technology as for fossil fuels and output a similar power. The complexity of hydrogen lies in its management, since it must be stored under pressure and being such a small molecule is prone to leaks.
The other application of hydrogen on the trips to the Moon was for electricity generation aboard the modules of the Apollo missions. In the same way that, with electricity, water can be separated into hydrogen and oxygen by electrolysis, combining hydrogen and oxygen in a fuel cell can produce electricity and water. Three fuel cells provided enough power to operate all the space probe instruments. In addition, the reaction product that combined hydrogen and oxygen was used in the cooling system of some devices and even as drinking water for the crew, although with some inconveniences such as bad taste and gas bubbles that are created in zero-gravity conditions.
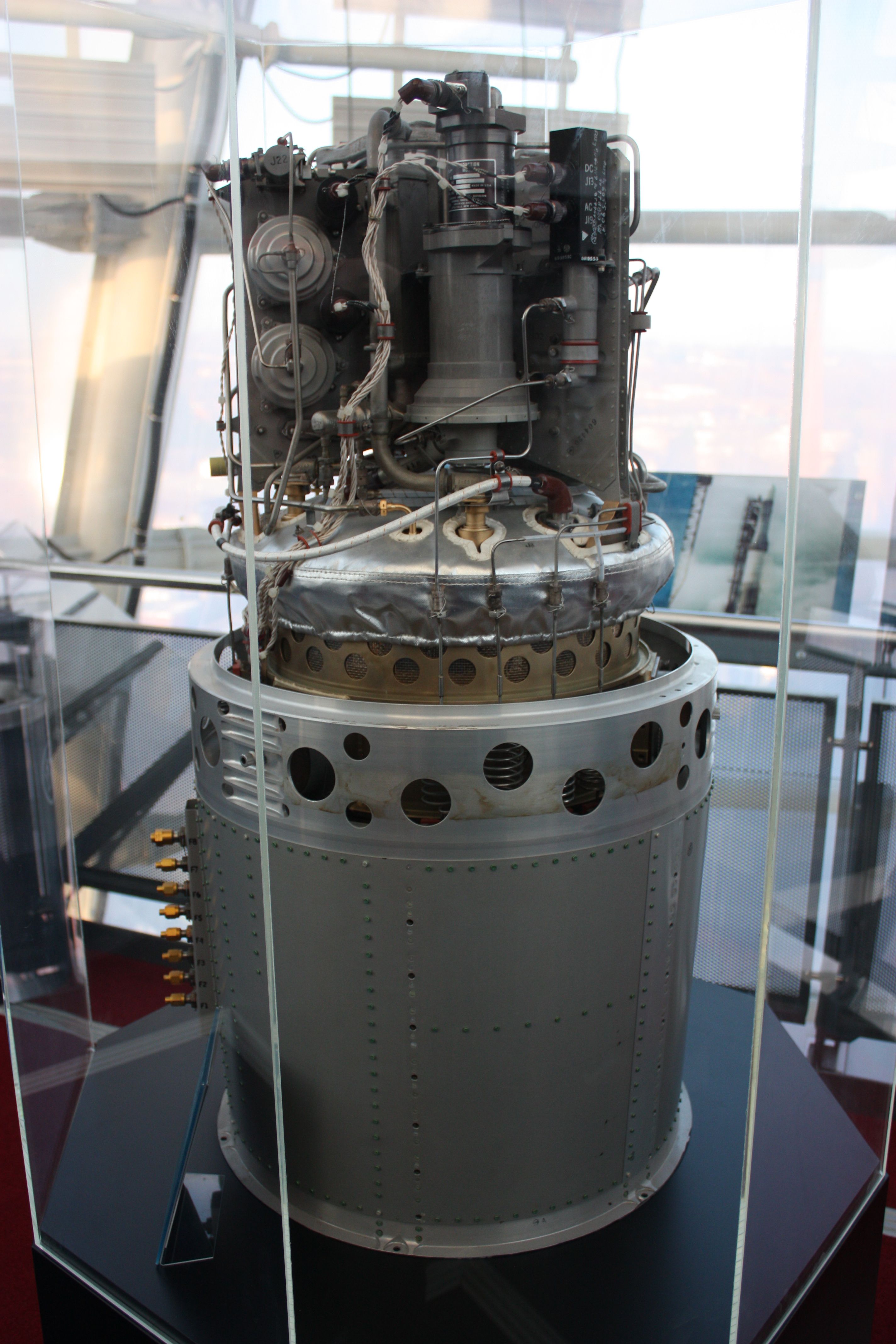
Hydrogen fuel cell from the Apollo missions. Source: Wikipedia. Author: James Humphreys – SalopianJames CC BY-SA 3.0.
Hydrogen and the energy transition
The role that hydrogen is expected to play in the energy transition contemplates both its use as fuel and for the direct electricity generation.
In terms of its use as a fuel, hydrogen can now be used mixed with natural gas up to a certain concentration without changing existing technology. The use of hydrogen mixed with natural gas in a CCGT to generate electricity or by injecting it directly to the gas distribution grid can help to reduce CO2 emissions and reduce the natural gas demand.
In its use for the direct electricity generation by means of chemical reaction with oxygen, its most visible application is in hydrogen vehicles. A car that is powered by hydrogen works with an electric motor, but unlike plug-in electric cars, the electricity needed to make it work is generated in the vehicle itself in a fuel cell were the hydrogen reacts with the oxygen in the air to generate electricity. Electricity powers the electric motor and the only waste is the water produced in the reaction.
The comparison between hydrogen vehicles and battery electric vehicles is common, and there is a very interesting debate about which of the two options should be chosen for the decarbonisation of transport. Although there are also those who advocate for the common sense of a coexistence of the two technologies in the future mobile pool.
Among the advantages of the hydrogen vehicle over that of batteries, autonomy, weight and refuelling time stand out. While the drawbacks include complexity, efficiency and, at least for the time being, the price and availability of refuelling points. From AleaSoft, it is expected that each technology will have its market share according to the use and needs of the vehicle. In everyday cars with average distances of less than 200 km, the battery electric car is perfectly suitable if it can be recharged at night or while parked. For vehicles that require more energy to make longer distances such as trucks, boats or airplanes, or vehicles where refuelling time is important, such as taxis, hydrogen will be a more affordable option.
But the key role of hydrogen in the energy transition according to AleaSoft’s specialists will be its combination with renewable energies. The disadvantages of an intermittent renewable energy source, such as wind or photovoltaic, can be solved using hydrogen as energy storage. When there is surplus of electricity or when the electricity market price is very low, renewable energy generation can be used to produce hydrogen. This hydrogen can be stored and converted into electricity in a fuel cell when the market price is more advantageous, or even sold for use as fuel or for refuelling hydrogen vehicles.

Hydrogen production
In order for hydrogen to efficiently help reduce CO2 emissions it is necessary that CO2 is not generated during its production. Generating hydrogen directly from water electrolysis is expensive because it is a process that requires a lot of electricity, so most hydrogen is currently produced from methane gas. In the steam reforming process, four molecules of hydrogen are produced from each methane gas molecule, but also a CO2 molecule is created.
But generating hydrogen by electrolysis does not imply that CO2 is not released. If the electricity used for electrolysis is not 100% of renewable origin, then there was CO2 emission at some point in the generation of hydrogen. It is here that the synergy between hydrogen and renewable energies has a key role to play. For example, a hydrogen production plant associated with a photovoltaic plant implies having renewable energy at a very low cost to produce hydrogen that is 100% free of CO2 emissions.
Hydrogen revolution + photovoltaic revolution
The future of hydrogen faces very interesting challenges that will lead to significant improvements in the efficiency of its use, as fuel and in fuel cells, and its production by electrolysis. Its nature as a renewable and non-polluting gas, positions it as an unquestionable winner against polluting and non-renewable fossil fuels.
According to AleaSoft, the hydrogen revolution will be complete if it is paired with the photovoltaic revolution because both compensate for each other’s deficiencies and are complementary. The ability to use hydrogen to store energy will supply the intermittency of photovoltaics and will mean not having to sell production when electricity market prices are low. For hydrogen, low-cost and renewable energy from photovoltaics is the key to having a gas that can gradually replace other fuels such as diesel, gasoline and finally natural gas.
Source: AleaSoft Energy Forecasting.
